Tokyo University of Agriculture and Technology Yoshino Lab.
Development of Single-Cell Technology
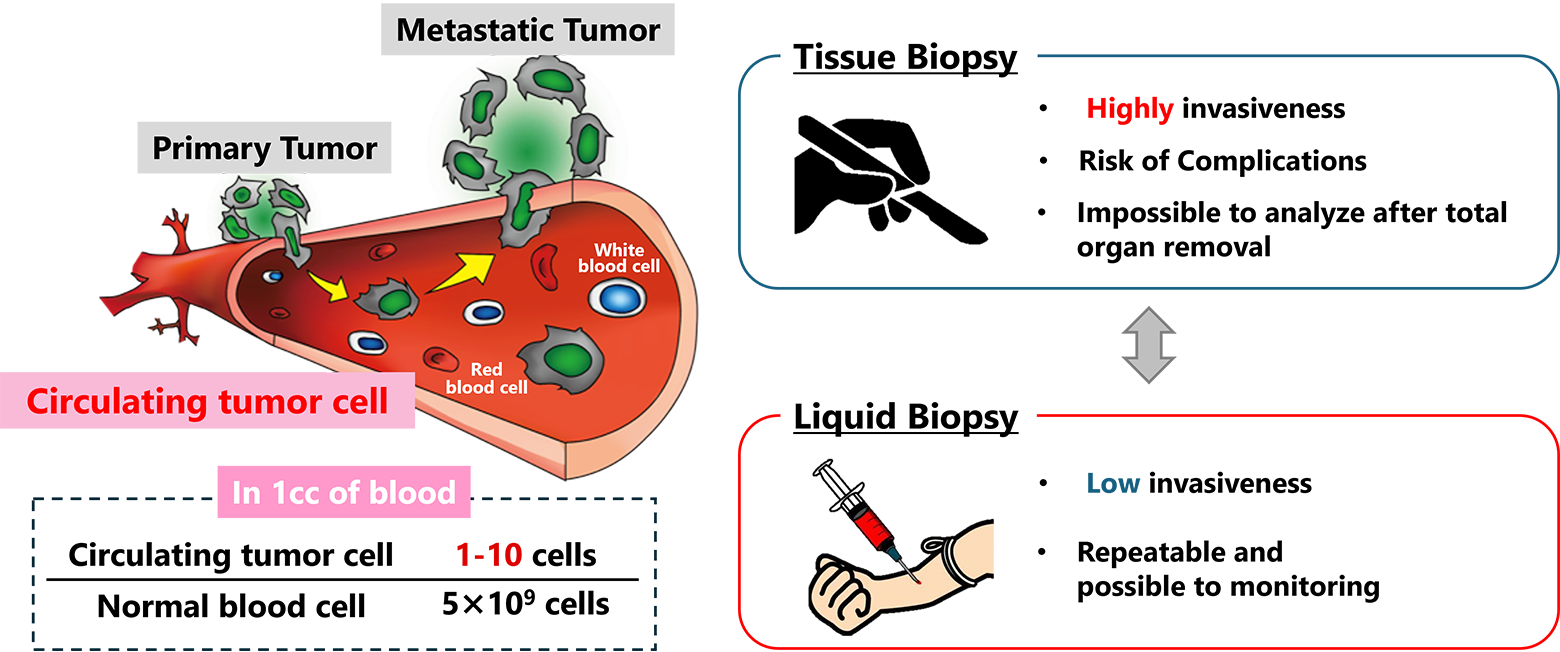
What is Circulating tumor cells (CTCs)
Cancer is a disease that often leads to fatal outcomes due to repeated metastasis. As a result, developing tools to predict metastasis is a critical aspect of cancer diagnostics. Traditionally, tissue biopsies, which involve directly sampling cancerous tissues, have been used for this purpose. However, this approach places a significant burden on patients and carries risks, including potential complications. To address these challenges, there is growing interest in minimally invasive techniques like liquid biopsy.
One of the key targets of liquid biopsy is circulating tumor cells (CTCs)—cells that break away from cancerous tissues, enter the bloodstream, travel throughout the body, and contribute to metastasis. CTCs are considered a promising biomarker, but they are extremely rare, with only 1 to 100 cells found among approximately 5 billion blood cells in a single milliliter of blood. This rarity underscores the need for advanced technologies capable of efficiently isolating and recovering CTCs from blood for detailed analysis.
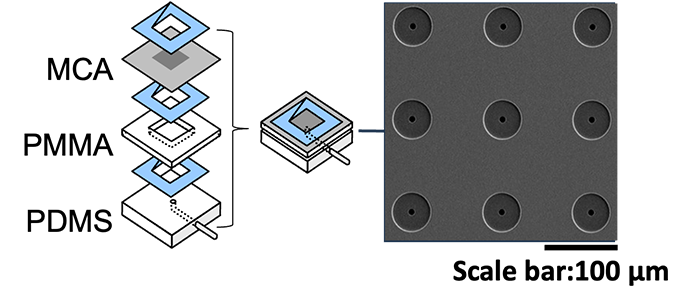
Technology of Collecting CTC Using Microcavity Array (MCA)
We have developed a filter-type device called a microcavity array (MCA), which consists of fine pores of several micrometers in diameter arranged at equal intervals, and by aspirating the cells, they can be densely arranged (arrayed). Our laboratory has found it possible to collect CTCs by using MCAs. As a result of studying the size, shape, and number of pores suitable for CTC collection, we have succeeded in developing MCA that can collect cancer cells with a collection rate of more than 95% from a mock sample of cancer cells mixed with blood from a healthy individual. We are currently developing an automated blood cancer cell collection device for practical use of this technology, and are conducting clinical trials in collaboration with medical institutions. We are also developing MCA to collect white blood cells and microorganisms.
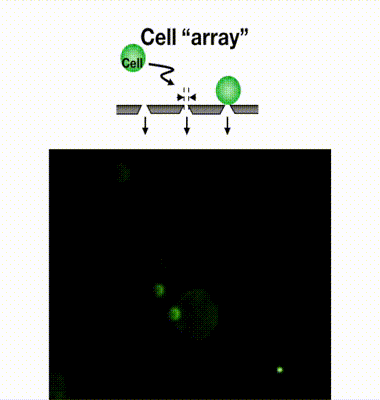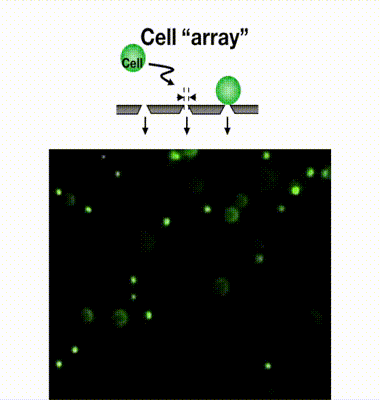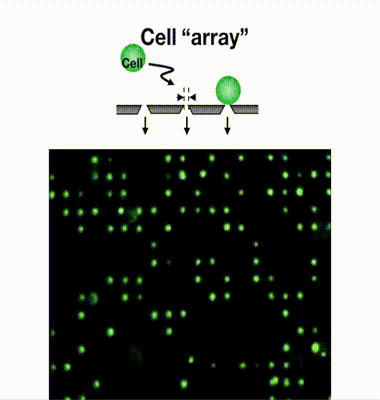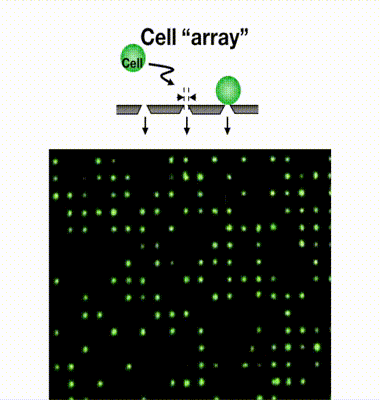

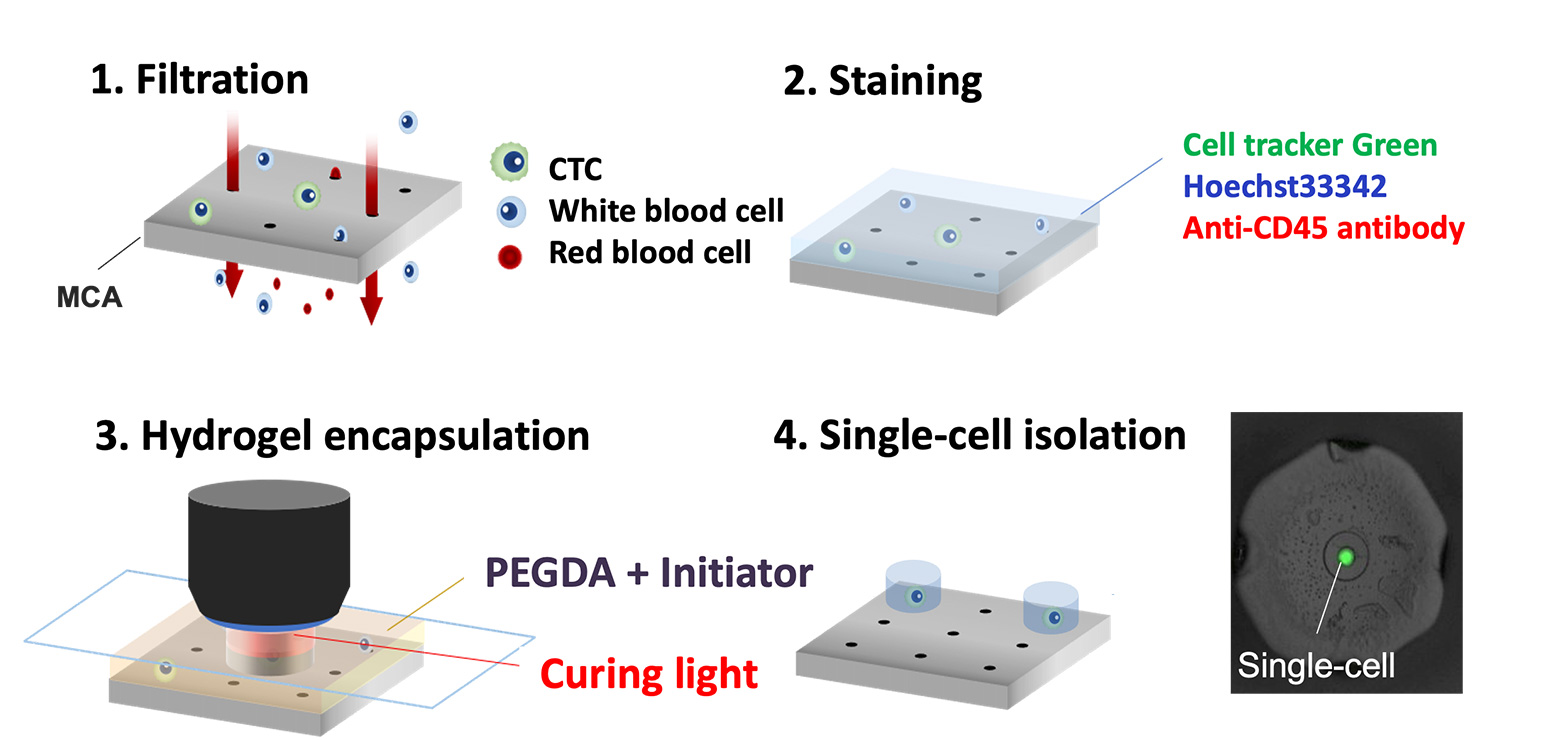
Single-cell isolation technology: Gel-based cell manipulation (GCM)
To thoroughly evaluate the characteristics of CTCs, it is crucial to isolate individual cells and analyze their genomes and transcriptomes. However, conventional methods often involve bulky equipment and complex procedures for single-cell isolation. Additionally, the extremely low abundance of CTCs makes it difficult to isolate them from MCA collections without significant loss.
To address these challenges, our laboratory developed an innovative technique called Gel-based Cell Manipulation (GCM). This method embeds cells in a gel that is large enough to be visually identifiable, making it easier to isolate CTCs recovered by the MCA. The GCM technique utilizes a photocurable hydrogel that solidifies when exposed to specific wavelengths of light, enabling the precise embedding of individual cells within the gel. This innovation allows for the accurate manipulation of single cells using tweezers. We are currently conducting proof-of-concept studies to demonstrate the separation and genetic analysis of CTCs from blood samples of cancer patients using this technology.
Furthermore, since this method eliminates the need for specialized equipment or advanced technical expertise, it offers significant potential for facilitating single-cell analysis and broadening its application to other cell types beyond CTCs.