Tokyo University of Agriculture and Technology Yoshino Lab.
Development of Magnetic Nanoparticle Library
Creation of membrane receptor-magnetosome complexes for drug discovery
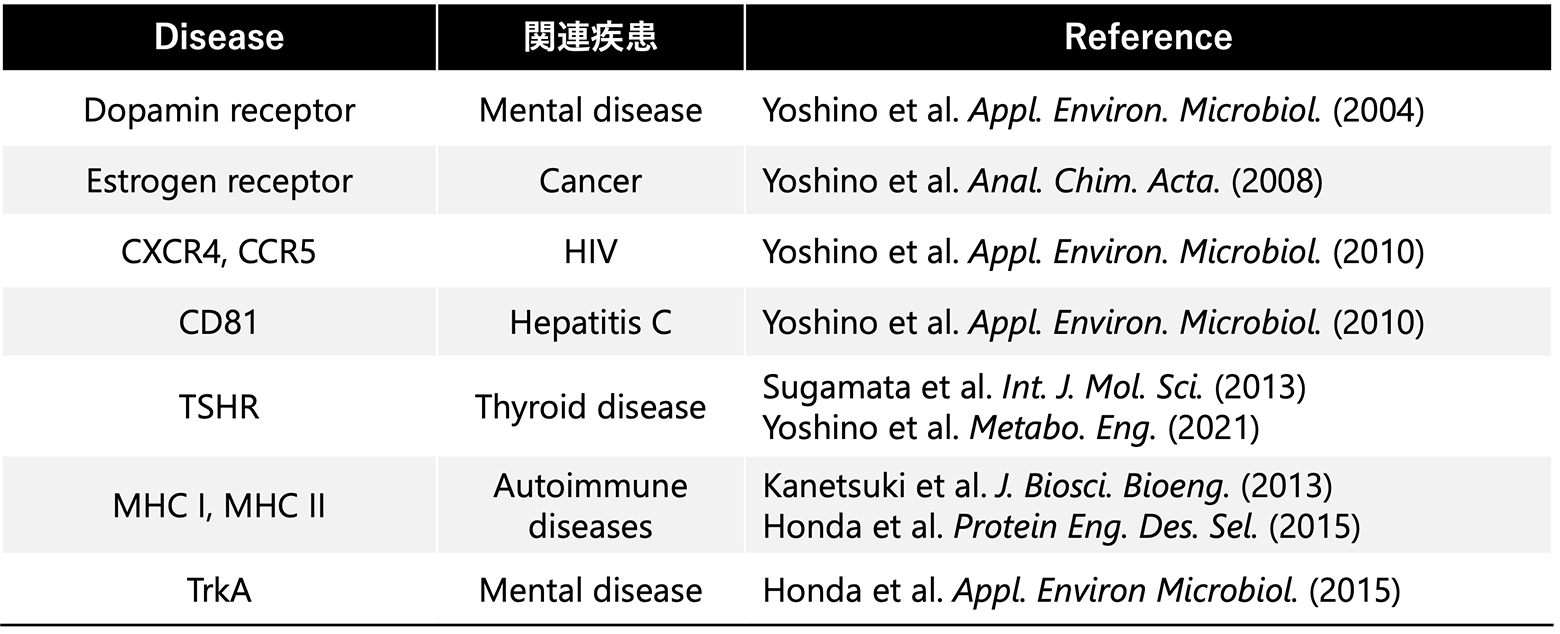
We have successfully expressed functional membrane proteins associated with various diseases. These membrane proteins are well-known as critical drug discovery targets. By utilizing membrane protein-magnetosome complexes, we anticipate the development of novel drug screening technologies.
To date, we have achieved the functional expression of several key membrane proteins, including CD81, a transmembrane protein involved in the infection mechanism of hepatitis C virus, thyroid-stimulating hormone receptor (THSR), a G protein-coupled receptor, and TrkA, the tyrosine kinase receptor related to mental diseases such as the Alzheimer’s disease and depression. Through this technological development, we have successfully produced the target membrane protein-magnetosome complex shown below.
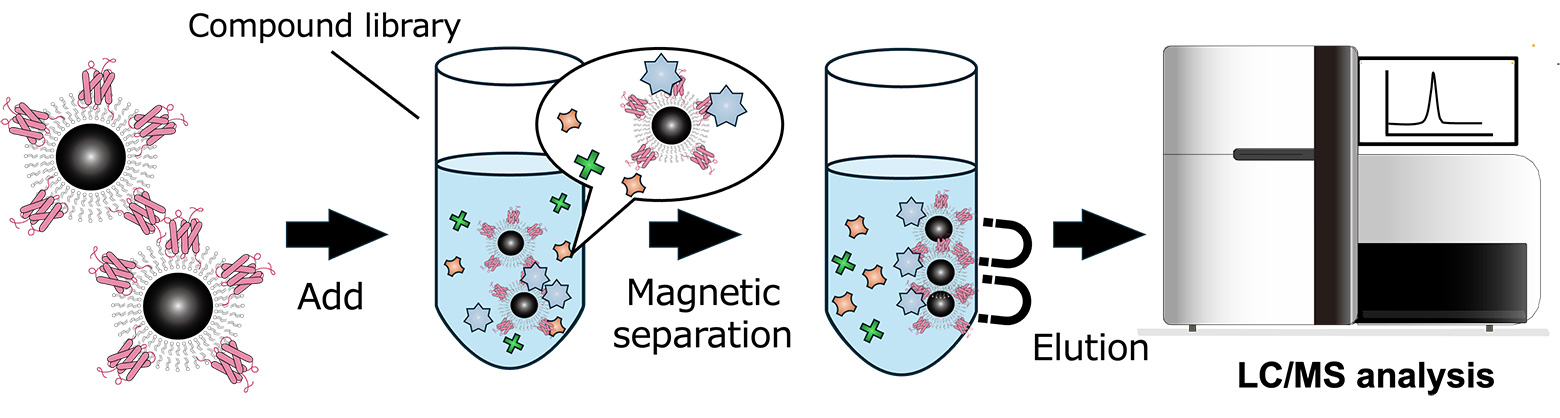
This target membrane protein-magnetosome complexes enable the efficient magnetic recovery of specific substances that bind to functional proteins from reaction solutions. This principle can be broadly applied across various fields, including the recovery of target compounds from environmental water samples and the isolation of target cells from blood.
Modulating magnetosome membrane lipids
We are developing various techniques to expand the utility of magnetosomes. One such innovation involves modifying the membrane lipids of magnetosomes. It is well known that the function of membrane proteins is significantly influenced by the lipid composition of their supporting membranes, and the lipid profiles of eukaryotic and prokaryotic organisms are distinctly different.
By altering the lipid metabolism pathways of magnetotactic bacteria, we successfully created magnetosomes with modified membrane lipid compositions. These modified magnetosomes exhibit notable features, such as enlarged magnetite crystals and enhanced functionality of expressed membrane proteins.
This advancement highlights the potential for tailoring magnetosome properties to optimize their performance in applications such as drug discovery, biocatalysis, and diagnostics, further broadening the scope of their utility.