Amyloidosis is a very mysterious disease. Diagnosis is not easy and sometimes even a professional pathologist misdiagnoses it. This page outlines the general disease and diagnostic methods of amyloidosis. If you need our diagnostic support, do not hesitate to contact us.
Amyloid
Most of the functional proteins present in living organisms are water-soluble globular proteins and are involved in transport and signaling. Amyloid is an insoluble fibrous protein formed by misfolding of these functional proteins. Under electron microscopy, it has a fibrillar shape with no branches and a diameter of about 7 to 15 nm. Amyloid has a cross-β-sheet structure in which β-sheets are regularly aligned perpendicular to the fiber axis, which is involved in physical resistance and staining properties.
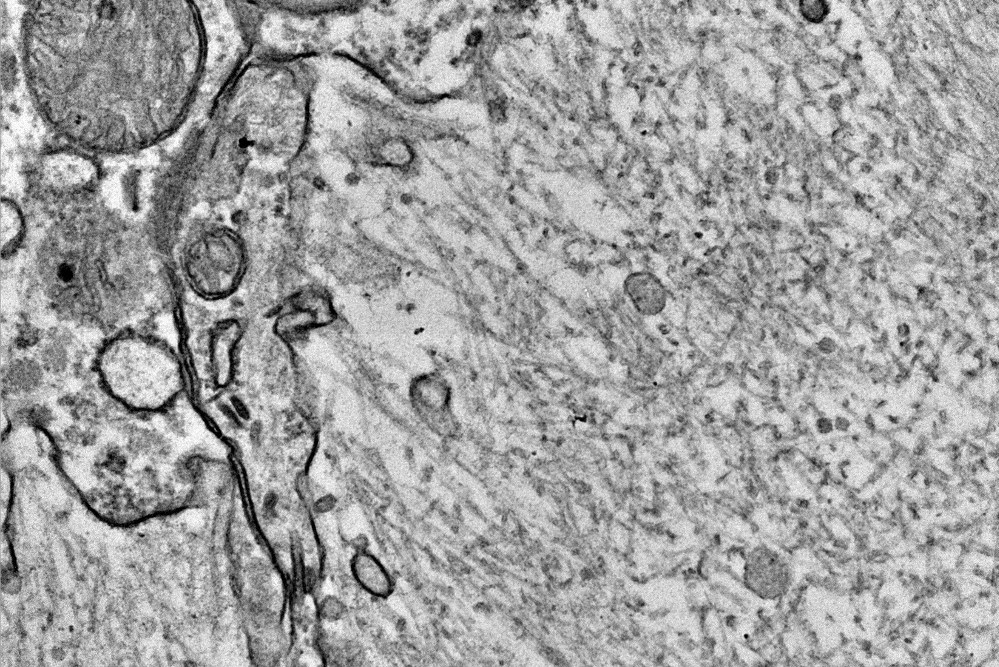
Electron microscopy of amyloid fibrils
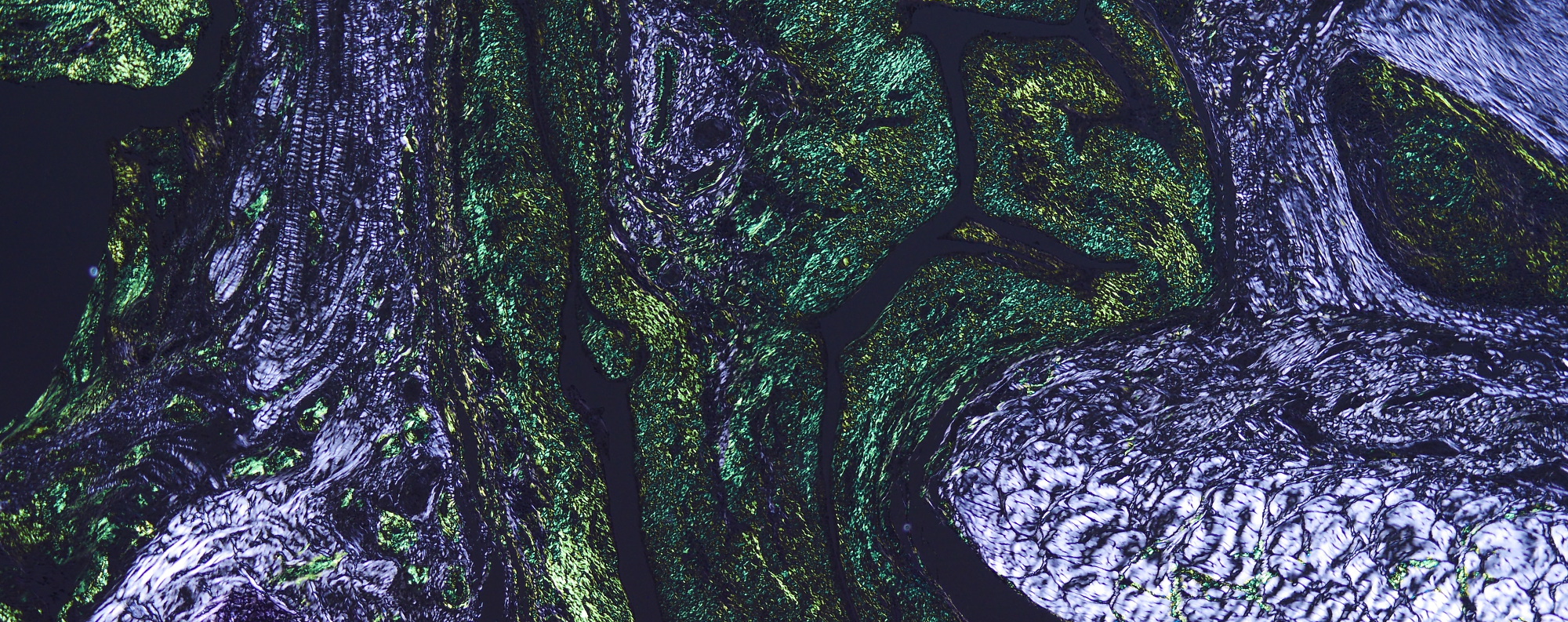
Amyloid is specifically stained with Congo red dye and exhibits orange~pink. And under polarized light, it exhibits yellow to green birefringence. The top image on this page is a Congo red polarization of chicken amyloid arthropathy, which is my favorite photo. It is a pleasure of amyloid research that we can sometimes encounter the breathtakingly beautiful emerald green.
The name amyloid comes from the Latin word "amylum" for starch. Amyloid has the property of turning black when immersed in an iodine solution, so it was named "amyloid" in the sense that it is starch-like.

Goat spleen soaked in an iodine solution (leopard print amyloid deposits)
Amyloid was named by Rudolph Virchow, the father of modern pathology. In 1854, he discovered the reaction of corpora amylacea with iodine in the nervous system and used the term amyloid. (However, it turns out that corpora amylacea in the brain is not amyloid). It is said that Virchow actually searched for starch in the human body and soaked various tissues in iodine solution.
Virchow proposed that amyloid is a substance similar to carbohydrates, which was denied by Schmidt in 1859. Schmidt noted that amyloid-deposited organs highly contain nitrogen and claimed that amyloid is a protein.
After that, "amyloid = protein" was determined by various analyzes, but the term amyloid (≓ starch) continued to remain. This may be due to the great influence of Virchow, and the common use of iodine solutions for the diagnosis of amyloidosis.
Amyloidosis
Amyloidosis is a general term for progressive and intractable diseases caused by local or systemic amyloid deposition. Amyloidosis is classified according to the type of precursor protein. According to the classification by the International Society of Amyloidosis in 2020, human amyloidosis is classified into 36 types and animal amyloidosis is classified into 10 types (actually, there are a few more types: the table below). Systemic amyloidosis occurs when the precursor protein is serum protein, and local amyloidosis occurs when it is produced locally.
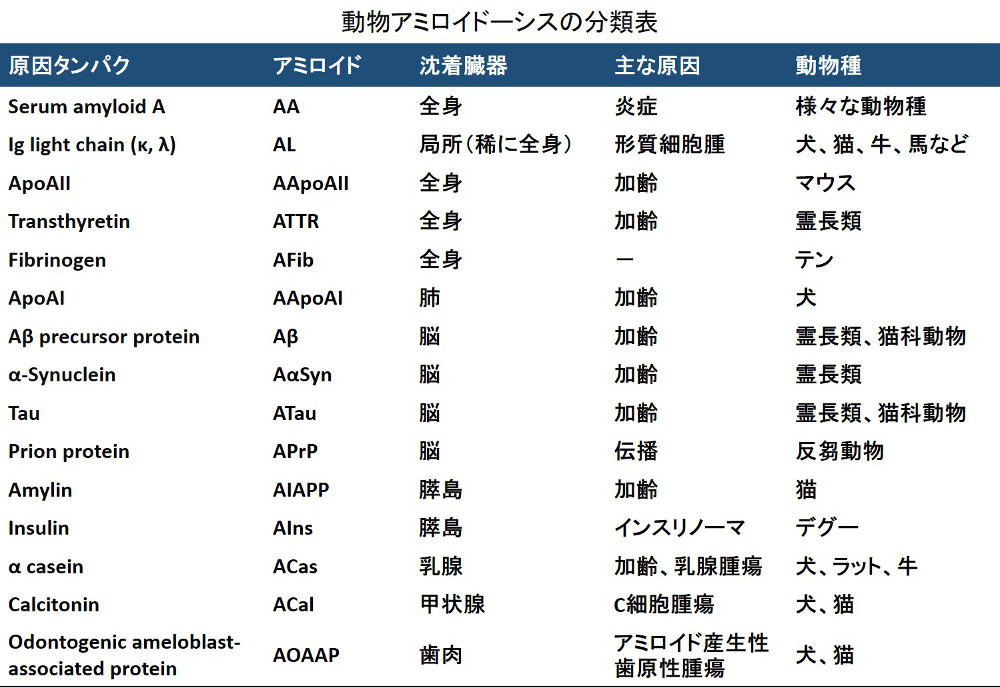
Occasionally, we see statements that divide animal systemic amyloidosis into primary (AL) and secondary (AA), but this is not accurate.
First, most of the systemic amyloidosis in animals is AA amyloidosis, and systemic AL is a very rare disease. AL amyloidosis in animals is observed as tumor-associated localized amyloidosis that is "secondary" to plasmacytoma. On the other hand, AA amyloidosis is usually reported to be secondary to inflammatory diseases, but idiopathic and familial one is also common depending on the animal species. For this reason, it is nonsense to classify amyloidosis into primary and secondary, and it is customary to classify by the precursor protein.
Histopathological diagnosis
A definitive diagnosis of amyloidosis is made by histopathological analysis. Therefore, it is very important to suspect amyloidosis first by clinical examination. However, there are many atypical cases of amyloidosis, and it is not uncommon to observe tissues and discover them by accident.
Iodine solution can be used to diagnose organs with a large amount of amyloid deposited at autopsy or meat inspection. Slice the organ into 5mm thick and immerse them in the iodine solution, and the area of amyloid deposition will turn black. If the organ is low in glycogen, such as the kidney, amyloid can be detected with particularly high contrast.

Detection of renal amyloid by iodine
Once amyloidosis is suspected and a biopsy or autopsy is performed and tissue sections are prepared, a definitive diagnosis can finally be made. In textbooks, amyloid is often described as "a weakly acidophilic homogeneous unstructured material deposited in intercellular or tissue spaces". But in fact, there are many exceptions.
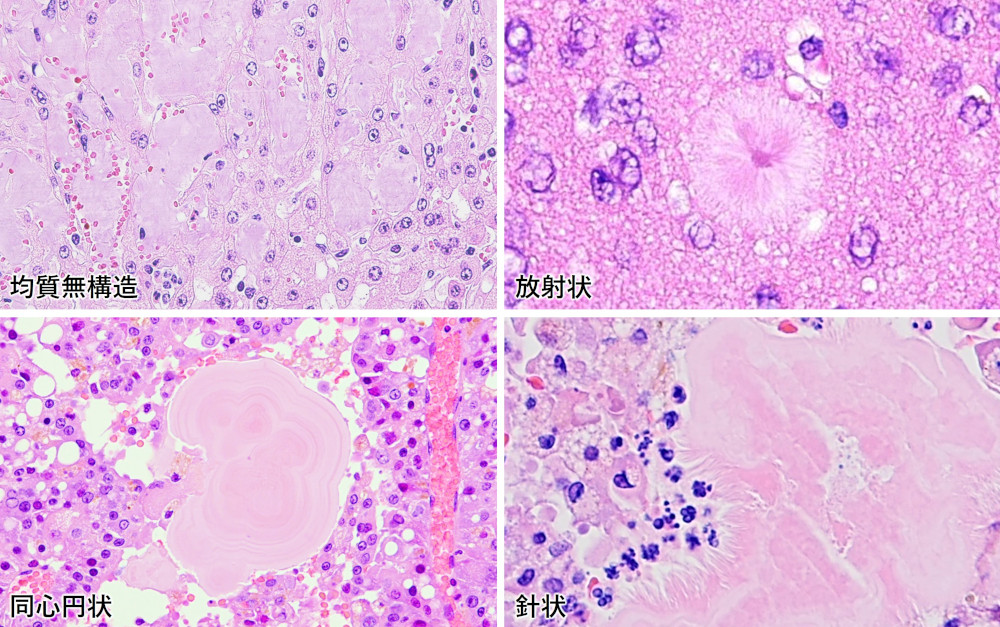
Various forms of amyloid deposition
If you have found amyloid-like deposits on the HE stained specimen, the next step is Congo red staining. In vivo amyloid is always positive for Congo red staining, and there are no exceptions to this rule. In pathology, reactivity to Congo red is one of the most important definitions of amyloid.
It is very important to note, however, that not all amyloid stains bright red with Congo red. The staining of Congo red depends on the precursor protein, the animal species, the deposition site, and the condition of the specimen.
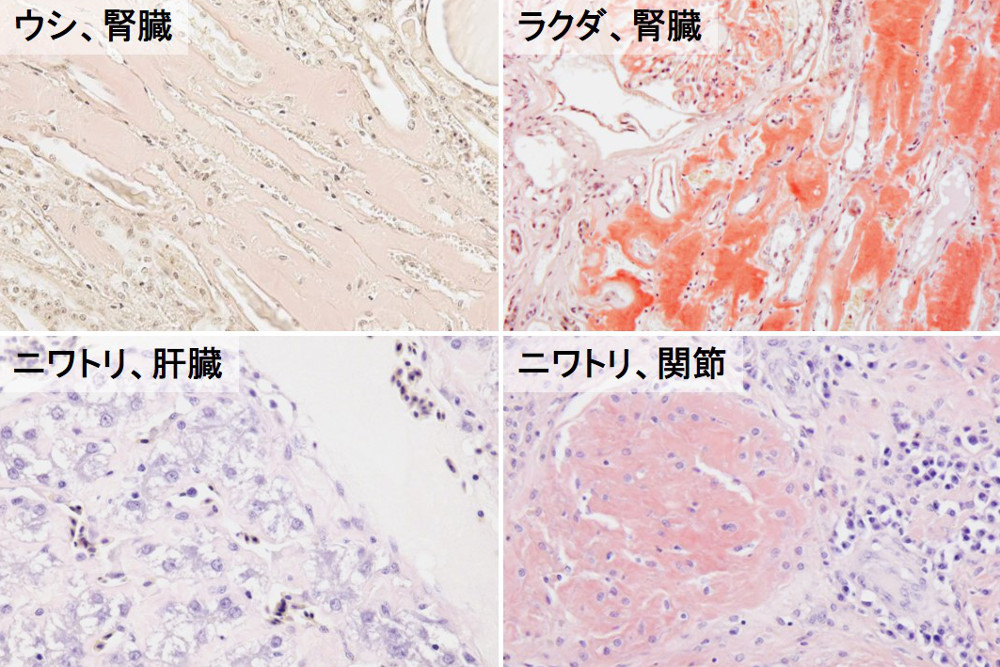
The above figures are all Congo red histological images of AA amyloidosis cases. The staining of Congo red varies so much between cattle and camels, even though they have the same AA and the same even-toed ungulates.
The lower part of the above figure shows amyloid deposited in the joints and liver of the same chicken. The amyloid deposited in the connective tissue of the joint is stained vividly, whereas the amyloid deposited in the Disse's space of the liver is only faintly positive.
The staining principle of Congo red is thought to be the binding of Congo red molecules using the regularly aligned grooves on the surface of amyloid fibrils. However, since Congo red has two sulfone groups in the molecule, its staining properties are actually affected by the amino acid composition in the protein. In other words, amyloid with few positively charged amino acid residues (R, K, H) will have greatly reduced staining, while proteins rich in these amino acid residues may be stained with Congo red even if they do not have a fibrillar structure.
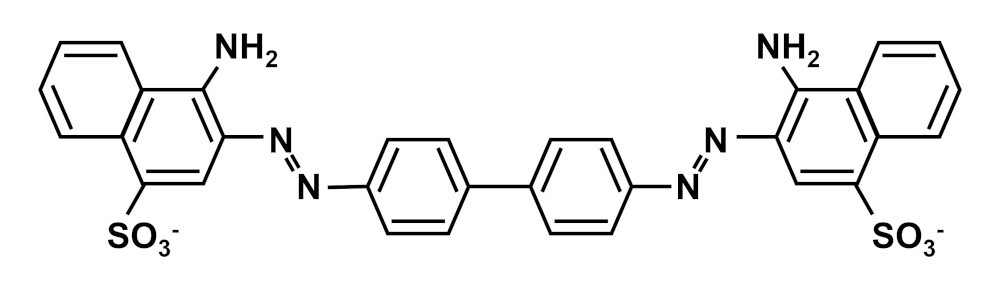
Chemical Structure of Congo Red
Experience has shown that in many animal species, AA tends not to stain Congo red. In contrast, AL and AOAAP are very strongly stained. As mentioned earlier, it is not easy to find systemic amyloidosis other than AA. I get very excited when I see a case of systemic amyloidosis that is strongly dyed Congo red.
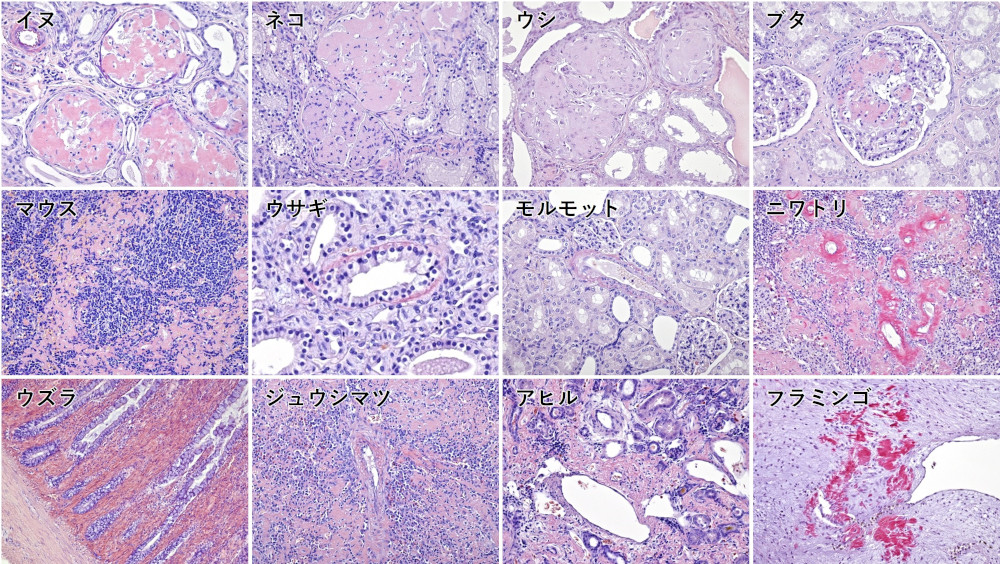
Congo red staining properties of AA in various animals
The staining characteristics of Congo Red must be well understood in order to make an accurate diagnosis. Other points to note about Congo Red are summarized in the column below.
Diagnosis by Congo red staining has been the gold standard for amyloidosis diagnosis for many years. However, there are some pitfalls to be aware of. This column describes "non-specificity of Congo Red" and "permanganate treatment".
Congo red dye often detects fibrillar proteins other than amyloid. Please see the following Congo red polarized light observation image of porcine liver.
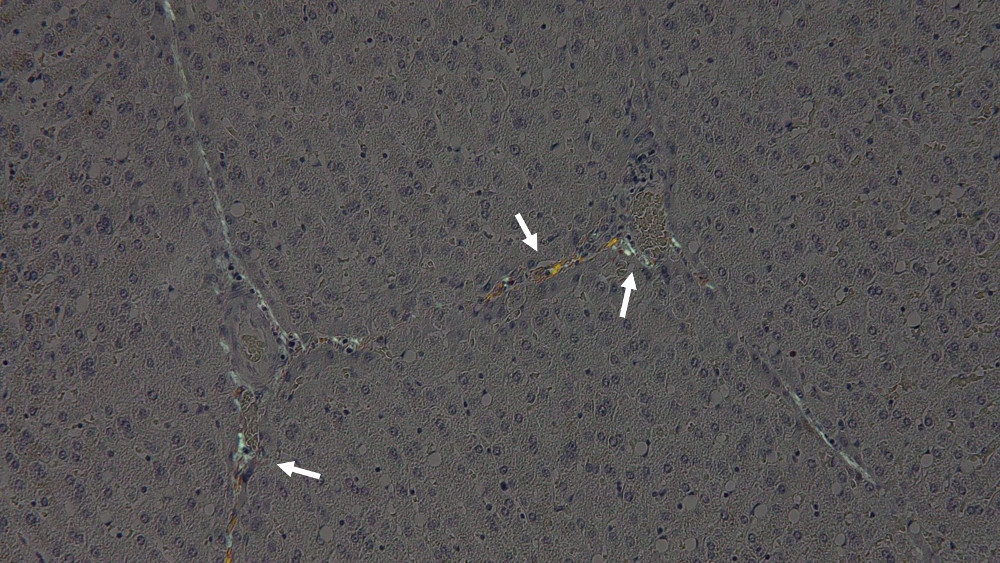
This case is negative for amyloidosis, and the green glow of the arrow is actually collagen fibers. Congo red staining often produces false positives due to factors such as differences in animal species, staining solution conditions, and lack of sorting. Also, as a characteristic of Congo red itself, collagen and elastin co-stain in the first place. If you rely on Congo red from the beginning, you will be misled by such non-specificity. HE staining should first be used to determine the location and histology of amyloid deposition, and Congo red staining should be used only as an adjunct to HE diagnosis.
Next, I would like to talk about potassium permanganate treatment.
Permanganate treatment was once used as an orthodox method to differentiate AA and AL. Perhaps some laboratories are still using it for definitive diagnosis. This method takes advantage of the fact that AA loses its affinity for Congo red by permanganate treatment, but there are exceptions.
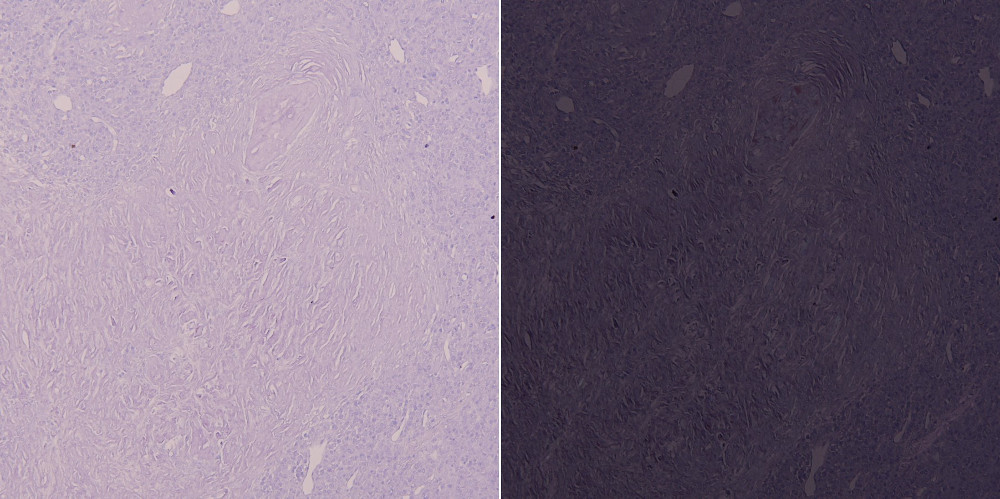
Left: Bright field, Right: Polarized light
The photo above shows a specimen of feline AL amyloidosis associated with plasmacytoma, treated with permanganate and stained with Congo red. As you can see, the polarization has disappeared. Even if the specimen is not AA, it may be intolerant to permanganate treatment depending on the condition of the specimen, the type of amyloid, the site of deposition, and the animal species. Conversely, some animal species, such as mice, are resistant to permanganate treatment even if they are AA. In recent years, the number of amyloid classifications has increased, and the use of potassium permanganate treatment has become meaningless.
Immunohistochemistry
Once amyloid can be detected by Congo red staining, it is time to identify the causative protein. Currently, amyloid identification in many institutions is mainly done by immunohistochemistry. However, while immunohistochemistry is easy to use, it has several problems.
In most cases, immunohistochemistry requires the purchase of commercially available antibodies. However, most of the commercially available antibodies target human proteins. Anti-bovine and anti-canine antibodies are scarce (and very expensive if they are available), and for minor animal species, commercially available specific antibodies are unlikely to be available.
For this reason, in the veterinary field, immunohistochemistry is often replaced by anti-human antibodies. However, of course, the amino acid sequences of most proteins differ between humans and animals.
For example, Aβ42, which forms senile plaques in Alzheimer's disease, has 100% homology between humans and chickens, and anti-human Aβ monoclonal antibodies can be used. On the other hand, calcitonin, the cause of thyroid amyloidosis, has a homology of only 46%, and cross-reactions are unlikely even with polyclonal antibodies. Even if it is stained, proving its specificity will be a painstaking task.

We prefer to use monoclonal antibodies as much as possible for immunohistochemistry because of their lot-to-lot stability and low non-specificity, but polyclonal antibodies are often the first choice in our laboratory, which handles a wide variety of animal species. Of course, we purchase antibodies after predicting the epitope, but even so, it is not uncommon to buy antibodies and find that they do not stain.
For these reasons, the identification of amyloidosis in animals, especially in rare animals, is not an easy task. Furthermore, in AL amyloidosis, the precursor protein, immunoglobulin, has variable regions, and other amyloids often have amino acid mutations and fragmentation. When dealing with such cases, it becomes very difficult to determine a positive or negative result by immunohistochemistry. If the amyloid encountered is a novel causative protein, immunohistochemistry is completely out of its depth.
Mass spectrometry
Mass spectrometry is a way to overcome the problems of immunohistochemistry described above.
In our laboratory, amyloid recovered from formalin-fixed paraffin-embedded (FFPE) tissue sections is mainly tested. Ideally, we would use a laser microdissection device to accurately collect the target tissue, but unfortunately, this is not a common device that is available everywhere. If the target amyloid deposit is relatively large (1 area >30,000 μm2 ), it can be recovered using a needle under a stereomicroscope.

Manual dissection using a stereomicroscope
Since FFPE sections are cross-linked by formalin, they are first boiled with surfactant to break the cross-link. The FFPE sections are then treated with enzymes such as trypsin and subjected to LC/MS/MS. The obtained MS/MS data is then used for database analysis using software such as Mascot to clarify the protein composition in the tissue.
λ light chain detected in amyloid deposits of plasmacytomas
The advantage of mass spectrometry is that it provides direct evidence of the presence of proteins in the target tissue. Also, unlike immunohistochemistry, which detects only a few proteins, mass spectrometry can comprehensively detect the protein composition in a sample. Furthermore, by using a database that covers a variety of animal species, we can overcome the problem of species specificity that immunohistochemistry has.
We have successfully analyzed amyloid in various animal species such as dogs, rats, squirrels, wildcats, and quails using mass spectrometry. Most of them could not be diagnosed by immunohistochemistry alone.
In the medical field, mass spectrometry is becoming the new standard for the diagnosis of amyloidosis, but in the veterinary field, it is still not a major analytical method. As mentioned above, mass spectrometry is a highly useful analytical method for animal studies that can overcome the immunohistochemistry problems faced by veterinary pathologists. We hope that this web page will be of help to those who are getting started with mass spectrometry.
Diagnostic support for animal amyloidosis
In recent years, the diagnosis of amyloidosis has become more and more difficult due to the increasing segmentation of the disease type. In this laboratory, we can diagnose amyloidosis in any animal species as part of our research. The main methods of analysis are immunohistochemistry and mass spectrometry. If you suspect amyloidosis or want to identify the causative protein, please do not hesitate to contact us.
For amyloidosis that cannot be identified by immunohistochemistry, mass spectrometry is an effective method of analysis. Although unfixed samples are more reliable, analysis of FFPE samples is also one of our specialties. In our laboratory, we use LC-MS/MS to analyze amyloid recovered from FFPE sections by various methods. Theoretically, we can diagnose all types of amyloidosis.
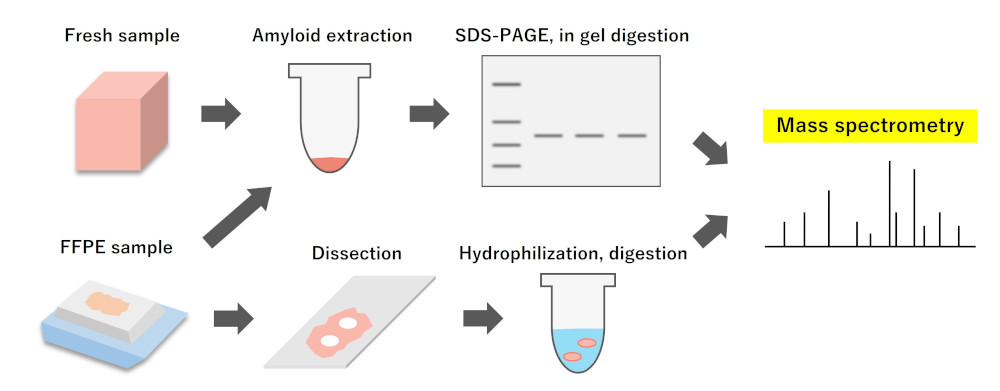
Identification of amyloid by mass spectrometry
New animal amyloidosis identified by mass spectrometry