Energy conversion of biomass with polar ionic liquids under mild conditions and its analysis
We have developed novel polar ionic liquids (ILs) which dissolve cellulose under mild conditions. These ILs have carboxylate anions (Biomacromolecules, 2006, 7, 3295. Link) or phosphonate anions (Green Chem., 2008, 10, 44. Link). Since they dissolve cellulose without heating, they are suitable solvents for efficient energy conversion of cellulosic biomass. In addition, they extract cellulose from plant biomass under mild condition (Green Chem., 2010, 12, 1280. Link). Especially, 1-ethyl-3-methylimidazolium phosphinate achieved efficient extraction of cellulose from wheat bran due to its low viscosity (under 70 cP at room temperature) (Figure 1).
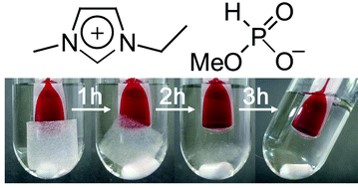
Figure 1. Dissolution of cellulose to ILs under mild condition.
As a latest result, tetrabutylphosphonium hydroxide (TBPH) was found to be an excellent solvent for cellulose (Chem. Commun., 2012, 48, 1808. Link). Surprisingly, 20 wt% of cellulose was dissolved into the solvent within 5min. As more interesting point of TBPH, it dissolves cellulose with a certain amount of water, resulting in biomass processing of "wet" biomass.
We also have focused on polar ILs which have dual functions; high polarity and hydrophobicity (Phys. Chem. Chem. Phys., 2013, 15, 4066. Link, Phys. Chem. Chem. Phys., 2013, 15, 14941. Link). ILs composed of phosphonium cations and phosphonate anions show high polarity and immiscibility with water. They have a potential to overcome critical problems of ILs, i. e. the separation difficulty of products from ILs.
Development of analytical methodologies of cellulose in polar ILs is also very important. Since polar ILs are considerably viscous, it is extremely difficult to apply polar ILs into analytical systems. However, we successfully applied polar ILs into HPLC as mobile phase by designing the low viscous polar IL and searching suitable measurement conditions. We named the system "HPILC" (Chem. Commun., 2011, 47, 1994. Link). HPILC clarifies molecular weight distribution (MWD) of many kinds of cellulose. Moreover, we succeeded to conduct direct analysis of cellulose depolymerization in polar ILs using HPILC. The analysis not only estimates of depolymerization quantitatively but also reveals mechanisms of depolymerization (Anal. Methods, 2013, 5, 3172. Link) (Figure 2).
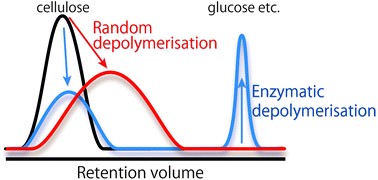
Figure 2. Differences of MWD of cellulose depolymerized by ultrasonication treatment or enzymatic reaction
Polymerized Ionic Liquids
We have reported polymerized ionic liquids (PILs) in 1998 for the first time in the world (Chem. Lett., 1998, 27, 751. Link). Although all of polyelectrolytes were brittle and stiff materials to deal with because of their high charge density, PILs were obtained as flexible films with low glass transition temperature (Tg). These advanced properties were gradually accepted by polymer scientists after more than five years of publication. These materials have different attractive points from those of ILs, and there are many attempts to seek the possibilities to apply PILs to many different research fields.
There are two major ways to prepare polymer electrolytes with relatively low-molecular weight ILs. One is gel-type polymer electrolyte composed of cross-linked polymer matrix and ILs and the other is the polymerized ILs. Former is proud of very high ionic conductivity that is comparable to that of pure ILs. However, the weak point is mobile ion species. In the gel-type polymer electrolytes, mobile ions are all component ions for the added ILs. Generally larger ions are used for the preparation of ILs, these component ions are not so useful for the electrochemical devices. The only exception was the capacitors or super-capacitors, where ion species are not the main issue but the ionic conductivity of the electrolyte layer is important. In spite of high ionic conductivity, there are a few drawbacks of these gel-type polymer electrolytes such as long-term durability and limit of ion species. Here we focused on the latter ones, so-called polymerized ILs.
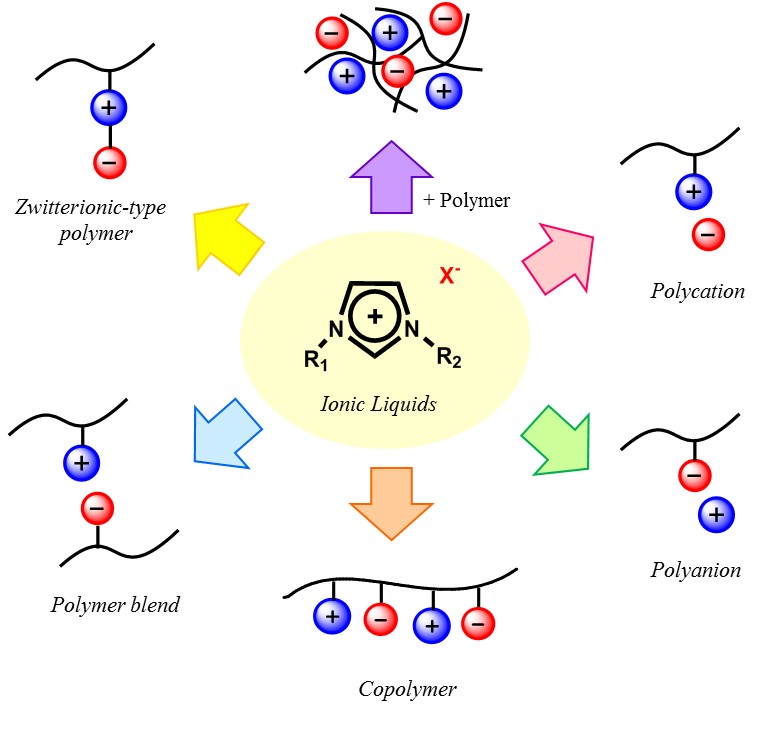
Figure 3. Strategies for the design of polymerized ionic liquids
Polymerizable cations such as N-viny-3-alkylimidazolium cations were synthesized and used to prepare polymerizable ILs. Polymerization of N-viny-3-alkylimidazolium salts was easily carried out as mentioned above. Since cations were fixed onto the polymer chains, these PILs were classified as anion-conductive polymers. As mentioned before, drawback of the polycation-type PILs is the drop of ionic conductivity. Addition of salts to these PILs improved the ionic conductivity. For example equimolar amount of LiTf2N was added to N-viny-3-alkylimidazolium Tf2N salt to considerably improve the ionic conductivity due to increase in the number of mobile ions.
Compared with cationic monomers, there are many anionic monomers which can form ILs. Much wider variety of structure of cations is the reason why many anions have chance to form ILs. In other words, it is possible to find suitable cations having adequate properties to form anionic monomers. Polymerization of ILs composed of anion monomers and cations gave polyanion type ILs in which only cations were mobile. Similar to previous study, simple polymerization of ILs composed of anion monomers and cations was quite easy to get solid polymers, but their ionic conductivity dropped considerably as compared with that of monomeric ILs. Spacer chains were also introduced between polymerizable group and IL ion pair to improve the ionic conductivity after polymerization. As expected, the ionic conductivity of polymers was improved 20 times higher than that of the polymer without any spacers.
Polymerized IL gels are also interesting materials. Oligoethylene oxide diacrylates have frequently been used as cross-linker without elevating Tg of the polymers. Oligo(ethylene oxide) chains were used as spacer between polymerizable group and IL salt as well as cross-linker, expecting low Tg of the polymers. For example, tetra(ethylene oxide) diacrylates was added to the imidazolium-type polymerizable IL monomers, and the mixture was radical polymerized to prepare flexible and transparent cross-linked PIL film (Polymer, 2004, 45, 1577. Link) (Figure 4). They show high ionic conductivity of about 10-4 S cm-1 at room temperature due to low Tg. Cross-linkers with IL units were also designed to prepare thermally stable cross-linked PILs Polymer, 2005, 46, 11499. Link). Acryloyl groups were introduced into IL unit containing two imidazolium cations (so called Gemini-type ILs), and these were added as cross-linker to the imidazolium type IL monomers. For example, 1,4-bis{3-[2-(acryloyloxy)ethyl]-imidazolium-1-yl}butane bis[bis(trifluoromethane-sulfonyl) imide] was used as this kind of IL cross-linker. The polymerization gave cross-linked PILs with high ionic conductivity.
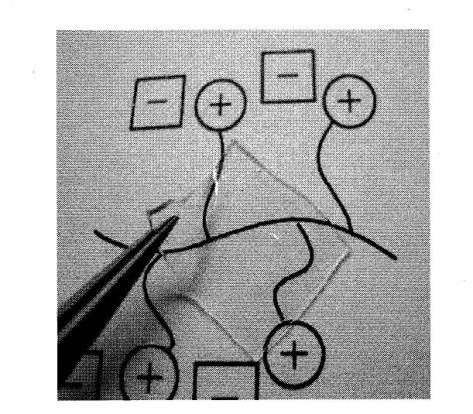
Figure 4. Cross-linked PIL film.
[Publications for polycation]
[Publications for polyanion]
Ionic liquids as biocatalytic media
Through it is difficult to dissolve proteins in ionic liquids (ILs), we have been reported some methods to realize dissolution of proteins in ILs remaining the original function. Poly(ethylene oxide) modification on the protein surface is one of the strong method to dissolve protein in ILs [1]. Additionally, structure design of polar ILs is enable to realize dissolution of cytochrome c (cyt.c) without any water molecule [2]. We also have been proposing "hydrated ionic liquid" as novel solvent for proteins [3]. In a hydrated IL, it is revealed that some proteins and DNAs are soluble and maintain the original higher-ordered structure. By using those methods, electron transfer reactions of dissolved proteins and enzymatic reactions were confirmed after dissolution and/or immobilization on an electrode in ILs [4].
[1] Poly(ethylene oxide)modification on the protein
The modification of poly(ethylene oxide) chains on the proteins is effective to dissolve the proteins into ILs. For cyt.c, at least 10 PEO chains with average molecular weight of over 2000 was required to solubilize it into ILs keeping the redox activity (Chem. Lett., 2003, 32, 450. Link). It was also confirmed that a suitable electrolyte was essential to realize the smooth electron transfer reaction of heme-proteins in ILs.
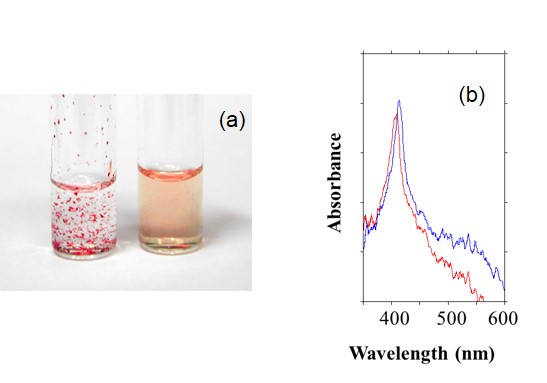
Figure 5. (a) Dissolution of cyt.c (left) and PEO-modified cyt.c (right) to [C4mim][Tf2N] and (b) OWG spectra of oxidized and reduced PEO-modified cyt.c.
[2] Structure design of polar ionic liquid
The solubility has been discussed with polarity parameters of the ILs (Biotechnol. Bioeng., 2012, 109, 729. Link). Both hydrogen bond basicity and dipolarity/polarizability of the ILs were confirmed to be influential factors to control the solubilization of cyt.c. Polar ILs such as 1-butyl-3-methylimidazolium chloride and 1-allyl-3-methylimidazolium chloride ([amim]Cl) solubilized cyt.c at 80℃, and the dissolved cyt.c was found to keep its redox activity in these ILs. The redox response of the dissolved cyt.c was detected in [amim]Cl up to 140℃.
![Solubility of cyt.c as the function of Kamlet-Taft parameters of the ILs (left) and OWG spectra of cyt.c in [amim]Cl (right)](img/fig3-1-2.jpg)
Figure 6. Solubility of cyt.c as the function of Kamlet-Taft parameters of the ILs (left) and OWG spectra of cyt.c in [amim]Cl (right).
[3] Hydrated ionic liquids as novel protein solvent
Hydrated ILs were prepared by adding appropriate amounts of water to hydrophilic ILs. In the hydrated ILs, water molecules were interact with ion and there is no fee water molecules in it with keeping the basic properties of ILs. Some hydrated ILs show excellent solubilizing ability for proteins (Biopolymers, 2010, 93, 1093. Link) and DNAs (Chem. Commun., 2012, 48, 5751. Link) with maintaining original higher-ordered structure. After dissolution in hydrated ILs, proteins showed excellent thermal stability and long-term stability compared with that in buffer solution (Biomacromolecules, 2007, 8, 2080. Link). Hydrated ILs, exhibiting cold crystallization behavior of water molecules in a certain range of water contents with DSC measurement, successfully dissolved cyt.c maintaining the original spin state of heme (Chem. Commun., 2013, 49, 3257. Link).
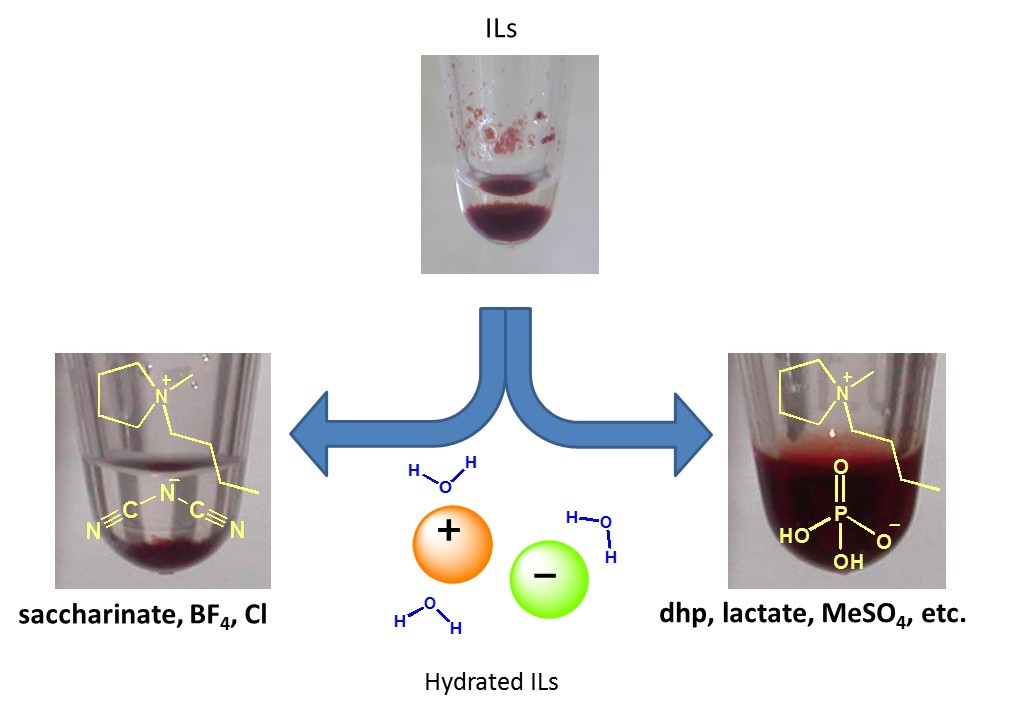
Figure 7. Differences of solubilities of proteins to hydrated ILs in terms ion structure.
[4] Enzymatic reaction and electron transfer reaction of proteins in hydrated ionic liquid
Hydrated choline dihydrogen phosphate (Hy[ch][dhp]) dissolved some metallo proteins (cyt.c, peroxidase, ascorbate oxidase, azurin, pseudoazurin and fructose dehydrogenase) without any modification (Biopolymers, 2010, 93, 1093. Link. These proteins retained the surroundings of the active site after dissolution in Hy[ch][dhp]. These metallo proteins were found to retain their activity in the Hy[ch][dhp]. Significant thermal stabilities of the enzymatic reaction were also observed (Electrochim. Acta, 2011, 56, 7224. Link).
![Cyclic voltammograms of fructose dehydrogenase-modified electrode with and without d-fructosein Hy[ch][dhp].](img/fig3-3-1.jpg)
Figure 7. Cyclic voltammograms of fructose dehydrogenase-modified electrode in Hy[ch][dhp] with and without d-fructose.
Temperature-driven reversible phase transition of ionic liquid/water mixtures
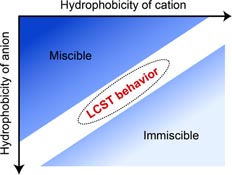
In 2007, we reported that ionic liquids having derivatives of amino acid anion and phosphonium cation showed lower critical solution temperature (LCST)-type phase transition with water [1], which underwent homogeneous phase on cooling and phase-separated upon heating. To acquire the design strategy for preparing ILs showing LCST-type phase transition with water, phase behavior was investigated in many IL/water mixtures. Our study clearly shows that total hydrophilicity of cation and anion is very important factor for designing ILs showing LCST-type phase transition with water [2]. From above knowledge, we proposed novel separation and purification media for proteins [3] and temperature-sensitive ionic liquid based electrolyte [4].
[1] LCST-type phase changes of a mixture of water and ionic liquids derived from amino acids, Angew. Chem. Int. Ed., 2007, 46, 1852-1855.
Link
[Abstract] Ionic liquids (ILs) derived from amino acids exhibit phase separation with a LCST after mixing with water. The phase-separation temperature of these mixtures depends on the ion structure and water content, and is lowered by an increase in the hydrophobicity of the ionic liquid.

[2] Material design of ionic liquids to show temperature-sensitive LCST-type phase transition after mixing with water, Australian J. Chem., 2011, 64, 1560-1567. Link
[Abstract] Phosphonium cations bearing different alkyl chains were coupled with several common anions so as to prepare ionic liquids (ILs) with diverse hydrophobicity. A few ILs were found to exhibit LCST-type phase transition after mixing with water. The phase separation temperature (Tc) of the IL/water mixtures depended strongly on the hydrophobicity of the component ions as well as mixing ratio. The number of water molecules per ion pair in the IL rich phase (mwater) increased dramatically upon cooling. The temperature dependence of this parameter was found to be useful to predict the possibility of the ILs to show the LCST-type phase behaviour after mixing with water. Since the value of mwater depended on the ion structure, especially on the hydrophobicity, the Tc was accurately set out by suitably mixing two ILs with different hydrophobicity.
[3] Selective transport of water-soluble proteins from aqueous to ionic liquid phase via a temperature-sensitive phase change of these mixture, Australian J. Chem., 2012, 65, 1548-1553. Link
[Abstract] When the mixture of tetrabutylphosphonium 2,4,6-trimethylbenzenesulfonate and water to show LCST-type phase transition was used, cytochrome c (Cyt.c) was found to be extracted from the water phase to the IL phase. Conversely, both horseradish peroxidase (HRP) and azurin remained in the aqueous phase. This selective extraction was comprehended to be due to the difference in solubility of these proteins in both phases. Finally we demonstrated selective extraction of Cyt.c from the 2 water-soluble proteins in aqueous solution via a LCST-type phase transition of IL/water mixture.

[4] Key factors to prepare polyelectrolytes showing temperature-sensitive LCST-type phase transition in water, Australian J. Chem., 2012, 65, 91-94. Link
[Abstract] Tetrabutylphosphonium styrenesulfonate and its homopolymer showed a LCST-type phase transition in water. As the hydrophobicity of these monomeric and polymeric salts affects phase behaviour, the phase transition temperature of the polyelectrolyte was changed by the introduction of monomers having different alkyl chain length on the phosphonium cations.

Design of liquid-crystalline systems based on ionic liquids and zwitterions
Liquid crystals are functional soft materials that self-organize into ordered states. In particular, liquid-crystalline materials having ionic liquid structures have been studied aiming for the application to a variety of fields including transportation materials and separation membranes. In Ohno laboratory, we have previously developed various functional ionic liquids. For example, a series of ionic liquids containing natural amino acids as anions, so-called amino acid ionic liquids, has been successfully prepared. Moreover, we have found that imidazolium-based zwitterions, in which imidazolium cation and sulfonate anion are covalently tethered, form homogeneous mixtures with an equimolar amount of bis(trifluoromethane)sulfonamide and the resultant mixtures act as ionic liquid like matrices. In this context, we have focused on the organization of these functional ionic liquids into ordered states and developed a new class of self-organizing materials by introducing liquid-crystalline properties into these novel ionic liquids. Their characteristic properties derived from their self-assembled nanostructures have been examined.
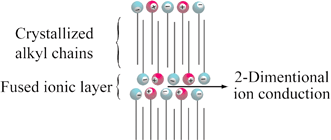
[1] Anisotropic ion conduction in a unique smectic phase of self-assembled amphiphilic ionic liquids, Chem. Commun., 2005, 1333-1335.
Link
[Abstract] Two types of thermotropic smectic phase and of anisotropic ion conduction were observed in an amphiphilic ionic liquid, N-ethyl-N9-dodecylimidazolium dodecyl sulfonate/lithium tetrafluoroborate mixture.
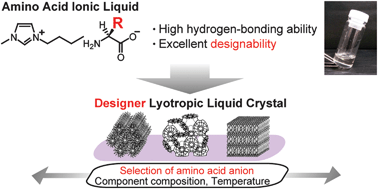
[2] Designer lyotropic liquid-crystalline systems containing amino acid ionic liquids as self-organisation media of amphiphiles, Chem. Comm., 2013, 49, 11746 - 11748. Link
[Abstract] Amino acid ionic liquids provide suitable media for lyotropic liquidcrystalline systems whose self-organisation behaviour is tuneable by the selection of amino acid anions.
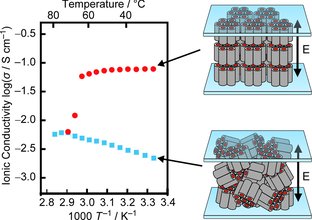
[3] Anisotropic proton-conductive materials formed by the self-organization of phosphonium-type zwitterions, Advanced Materials, 2011,23, 3071-3074. Link
[Abstract] A zwitterion/HTf2N complex system with columnar liquid-crystalline properties has been developed for a new type of proton-conductive materials. The columnar liquid-crystalline material shows efficient and anisotropic proton conduction behavior in the macroscopically oriented state.
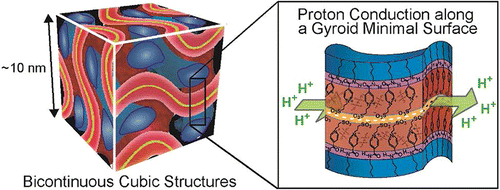
[4] 3D Continuous water nano-sheet as a gyroid minimal surface formed by bicontinuous cubic liquid-crystalline zwitterions, J. Am. Chem. Soc., 2012, 134, 11354-11357. Link
[Abstract] Co-organization of amphiphilic zwitterions and bis(trifluoromethanesulfonyl)imide led to the formation of bicontinuous cubic liquid-crystalline structures having 3D continuous hydrophilic gyroid minimal surface. The gyroid surface, incorporating a small amount of water, provided extremely thin but macroscopically continuous water nanosheet with a thickness of approximately 5 ?. The water nanosheet functioned as alignment free proton conduction pathway.
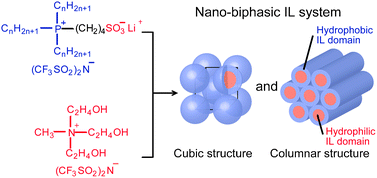
[5] Nano-biphasic ionic liquid systems composed of hydrophobic phosphonium salts and a hydrophilic ammonium salt, Chem. Commun., 2012, 48, 5271-5273. Link
[Abstract] A combination of a phosphonium-type-zwitterions-lithium bis(trifluoromethanesulfonyl)imide complex and a hydrophilic ammonium salt provides a nanosegregated liquid-crystalline matrix consisting of hydrophilic ionic liquid (IL) domains and hydrophobic IL domains.