Research Topic 1
Photoreaction mechanism of 1,8-diaminonaphthalene
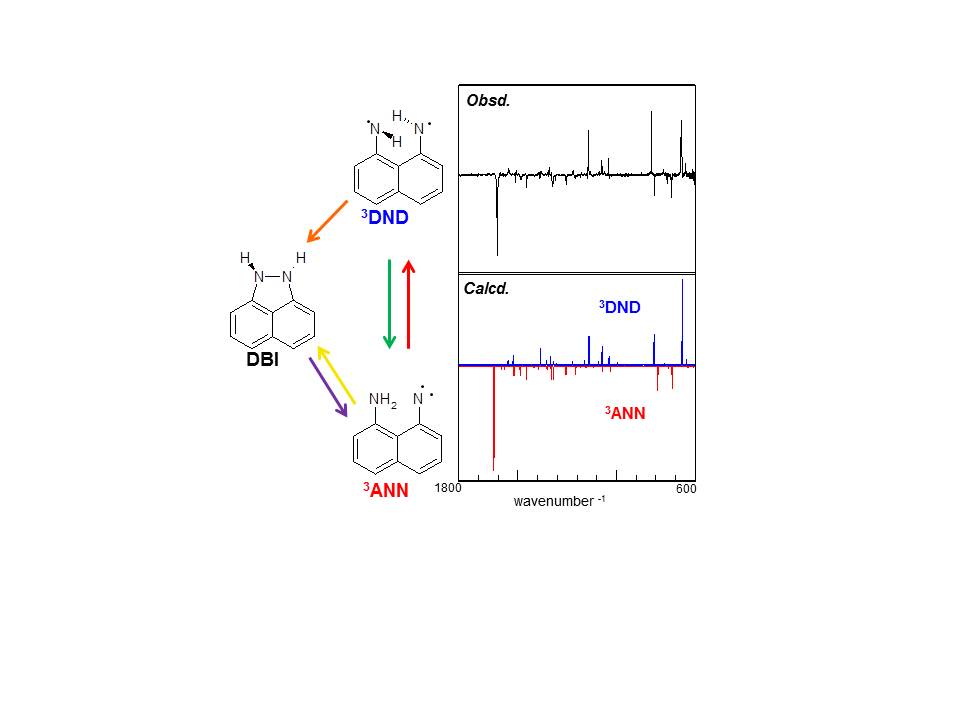
Nitrene is in general produced by photolysis of azido compound, but we identified triplet 8-amino-1-naphthylnitrene (3ANN) as a photoproduct of 1,8-diaminonaphthalene isolated in an Ar matrix. The reaction occurs upon 300 nm light irradiation (S1-S0 transition). It is more interesting that 3ANN isomerizes to 1,8-dihydro-1,8-naphthalenediimine (3DND) as a triplet diimine biradical and 1,2-dihydrobenz[cd]indazole (DBI) as a stable molecule upon visible light irradiation as shown in following scheme.
Takuya Okamura, Nobuyuki Akai, Munetaka Nakata, "Reversible Photoisomerization among Triplet Amino Naphthylnitrene, Triplet Diimine Biradical, and Indazole: Matrix-Isolation IR Spectra of 8-Amino-1-naphthylnitrene, 1,8-Naphthalenediimine, and 1,2-Dihydrobenz[c,d]indazole, Journal of Physcical Chemistry A, 121, 1633-1637 (2017). .